With the recent buzz about CRISPR clinical trials, we thought it was time for a comprehensive status update! It can be hard to keep track of all the different trials, especially since many of the same diseases pop up in the news as researchers test different treatment approaches. In this post, I’ll introduce you to the basics of clinical trials and then map out the current CRISPR-based trials from disease background to progress updates and what we really hope to learn from these trials.
Clinical trial basics
In the United States, the Food and Drug Administration (FDA) tests new disease treatments for safety and efficacy through clinical trials on patient volunteers. Early trials look at safety and side effects, while later trials test efficacy and compare new therapies with standard treatments.
The current trials using CRISPR therapies are all in the early stages. That means that even if they’re successful, they’re probably still a few years away from FDA approval.
Clinical trials are underway in three treatment areas: cancers, blood disorders, and eye disease. All current CRISPR clinical trials are intended to edit the specific tissues without affecting sperm or eggs, meaning no DNA changes can be passed onto future generations.
CANCERS
Disease background
Cancer refers to a group of diseases involving uncontrolled cell growth. Right now, CRISPR-based therapies are mainly aimed at treating blood cancers like leukemia and lymphoma.
Treatment strategy
T cells, better known as white blood cells, are covered in receptors that recognize other cells as safe or threatening. They patrol the body, killing foreign or dangerous cells. In CAR-T immunotherapy, researchers genetically engineer a patient’s T cells to have a receptor that recognizes the patient’s cancer cells, telling the T cells to attack.The immune system is highly regulated to avoid attacking healthy cells. Some T cell receptors work as “checkpoints” that determine whether an immune response occurs. When a T cell PD-1 receptor comes in contact with a molecule called PD-L1 on another cell, it communicates that it is a “safe” cell and the T cell leaves it alone.Cancer cells are clever and often cloak themselves in these molecular safety signals, tricking the patrolling T cells into ignoring them. Researchers are using CRISPR to edit the PD-1 gene in T cells to stop them from making functional PD-1 receptors so they can’t be fooled by cancer cells. This immunotherapy approach is known as a checkpoint inhibitor, and it is used in conjunction with CAR-T engineering to give T cells the greatest possible chance of eliminating cancer.In these treatments, researchers harvest T cells from a patient’s blood, and engineer them in a lab. This is a kind of ex vivo genome editing, because the genome editing occurs outside of the patient’s body. Ex vivo editing guarantees that genome editing tools only come in contact with the right target cells.
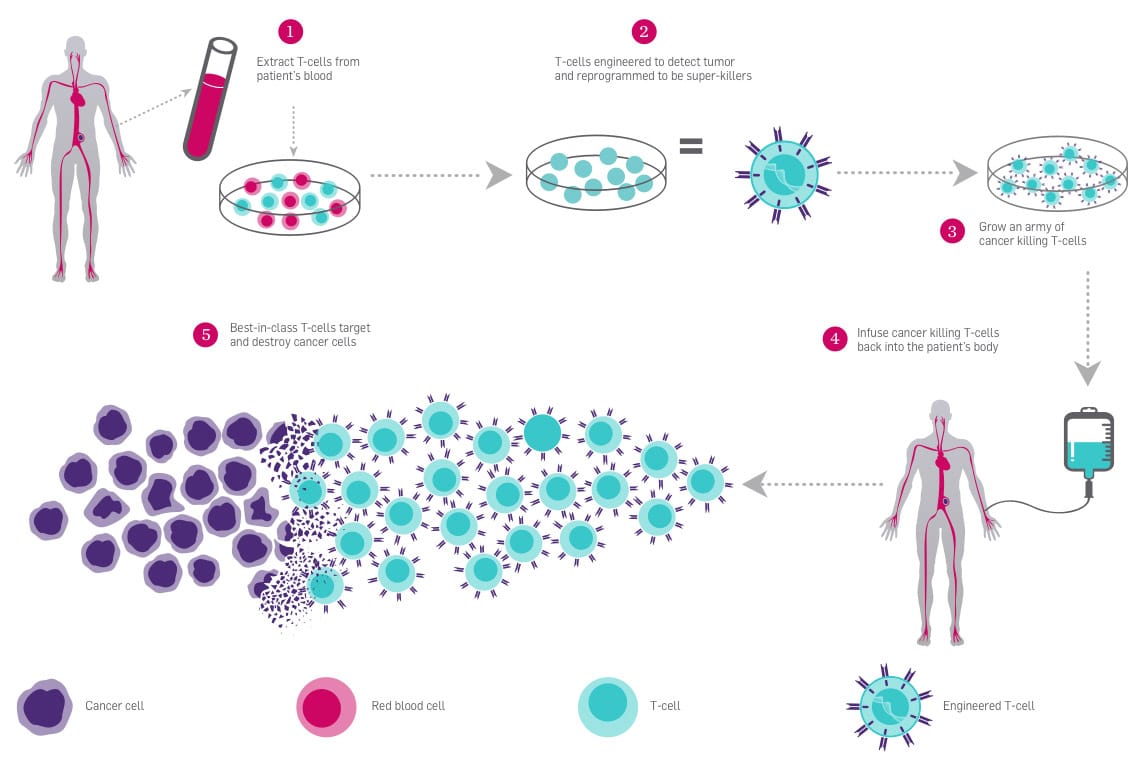
Overview of the immunotherapy process. Image courtesy of the Parker Institute.One of the big challenges for genetic therapies is delivery–that is, getting the medicine where it needs to be, and only where it needs to be. Blood cancers are some of the first targets for CRISPR therapies because the delivery is the most straightforward. Modified T cells can be delivered to the blood by IV, like getting a blood transfusion.
Current clinical trials
The first CRISPR-based therapy trial in the US combines CAR-T and PD-1 immunotherapy approaches. This study is currently underway at the University of Pennsylvania, in conjunction with the Parker Institute. Patient volunteers have late-stage cancers and few other treatment options. At least two patients have been treated, but we don’t know the outcomes yet.Clinical trials testing CRISPR immunotherapies to treat cancer may have begun as early as 2015 in China. Seven active or recruiting trials in China are listed on the US clinical trial database. While China is investing heavily in CRISPR, there are major concerns that clinicians are moving forward without proper clinical trial infrastructure, oversight, safety precautions, informed consent of patients, or research rigor. China has had repeated issues with fraudulent clinical trial data. Little data from active or completed studies has been released.
What to watch for
The FDA has already approved CAR-T therapies and PD-1 pathway inhibitors that don’t use genome editing. This is reason to be optimistic: the proof-of-principle work for these therapies has already been done!
That said, there’s still lots to look out for. While antibody treatments are available to disable PD-1, the efficacy of editing DNA to disable the PD-1 gene hasn’t been tested. In what percentage of cells will it work? Will genetic checkpoint inhibition work as well as current checkpoint-blocking treatments?These trials should also provide insight into broader questions about CRISPR-based editing: what sorts of changes will happen at the site of the DNA break that

















