Engineered Human Therapies
First Human Mini-Brain with Functional Blood-Brain Barrier Unveiled
This groundbreaking development by Cincinnati Children's researchers could accelerate drug discovery and therapeutic interventions for various brain disorders
[DALL-E]
In a groundbreaking achievement, researchers at Cincinnati Children's have developed the world's first human mini-brain that incorporates a fully functional blood-brain barrier (BBB). This milestone, detailed in the latest issue of Cell Stem Cell, holds promise for advancing the understanding and treatment of numerous brain disorders, including stroke, cerebral vascular disorders, brain cancer, Alzheimer’s disease, Huntington's disease, Parkinson's disease, and other neurodegenerative conditions.
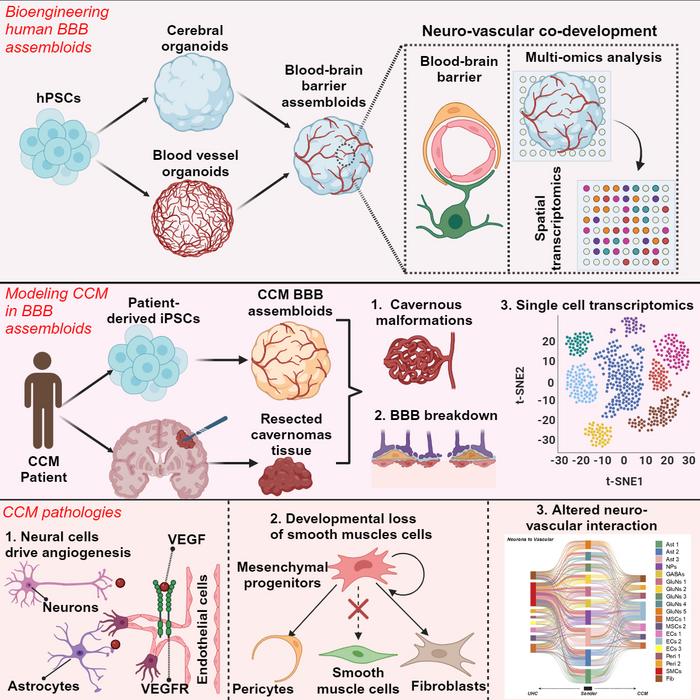
"Lack of an authentic human BBB model has been a major hurdle in studying neurological diseases," says lead corresponding author Ziyuan Guo, PhD. "Our breakthrough involves the generation of human BBB organoids from human pluripotent stem cells, mimicking human neurovascular development to produce a faithful representation of the barrier in growing, functioning brain tissue. This is an important advance because animal models we currently use in research do not accurately reflect human brain development and BBB functionality."
Understanding the Blood-Brain Barrier
Unlike blood vessels in other parts of the body, those in the brain have an extra lining of tightly packed cells that restrict the size of molecules passing from the bloodstream into the central nervous system (CNS). This barrier maintains brain health by blocking harmful substances while allowing essential nutrients through. However, it also prevents many potential medicines from reaching the brain, and its dysfunction is implicated in various neurological disorders.
"Now, through stem cell bioengineering, we have developed an innovative platform based on human stem cells that allows us to study the intricate mechanisms governing BBB function and dysfunction. This provides unprecedented opportunities for drug discovery and therapeutic intervention," Guo says.
Overcoming a Long-Standing Challenge
Around the globe, research teams have been striving to develop brain organoids—tiny, 3D structures that simulate early brain development. Unlike traditional flat cell cultures, organoids are self-assembling, spherical structures where cells interact as they would in a developing brain. Cincinnati Children’s has led in developing functional intestine, stomach, and esophagus organoids. Yet, creating a brain organoid with a functioning BBB remained elusive until now.
The new model, termed “BBB assembloids,” combines brain organoids that replicate human brain tissue with blood vessel organoids that mimic vascular structures. Initially, brain organoids measured 3 to 4 millimeters in diameter, while blood vessel organoids were about 1 millimeter. Over a month, these structures fused into a single sphere, slightly more than 4 millimeters in diameter, roughly the size of a sesame seed. These assembloids recreate many neurovascular interactions observed in the human brain, although they lack immune cells and connections to the body's nervous system.
The BBB assembloids can be grown from neurotypical human stem cells or those from individuals with specific brain diseases, reflecting various gene variants and conditions that affect the blood-brain barrier.
Initial Proof of Concept
To showcase the utility of these assembloids, the researchers used patient-derived stem cells to create models replicating key features of cerebral cavernous malformation, a rare brain condition marked by clusters of abnormal blood vessels. This genetic disorder compromises BBB integrity and increases stroke risk.
"Our model accurately recapitulated the disease phenotype, offering new insights into the underlying molecular and cellular pathology of cerebral vascular disorders,” Guo says.
Potential Applications
The researchers envision several applications for BBB assembloids:
Personalized Drug Screening: Patient-derived BBB assembloids could help tailor therapies based on individual genetic and molecular profiles.
Disease Modeling: The technology could accelerate the development of models for various neurovascular disorders, including rare and complex conditions.
High-Throughput Drug Discovery: Scaling up assembloid production could facilitate rapid analysis of whether brain medications can effectively cross the BBB.
Environmental Toxin Testing: BBB assembloids could offer a more accurate method for evaluating the effects of environmental pollutants and other chemicals.
Immunotherapy Development: By studying the BBB’s role in neuroinflammatory and neurodegenerative diseases, assembloids could aid in delivering immune-based therapies to the brain.
Bioengineering and Biomaterials Research: The model could support testing new biomaterials, drug delivery methods, and tissue engineering strategies.
“Overall, BBB assembloids represent a game-changing technology with broad implications for neuroscience, drug discovery, and personalized medicine,” Guo concludes.