The Compact CRISPR Enzyme Promising Breakthroughs in Genetic Medicine
Researchers unveil AsCas12f, a compact CRISPR enzyme poised to enhance precision gene editing and revolutionize genetic disorder treatments
Design Cells (Canva)
In a groundbreaking development published in Cell, scientists have unveiled a new, compact CRISPR-based gene-editing tool, AsCas12f. Engineered to be one-third the size of the widely-used Cas9, AsCas12f can be packaged more efficiently into adeno-associated viruses (AAVs), which serve as non-harmful carriers for introducing CRISPR enzymes into various cell types. This breakthrough enzyme has the potential to significantly enhance the efficiency and precision of gene editing, offering hope for more effective treatments for patients with genetic disorders.
Professor Osamu Nureki, from the Department of Biological Sciences at the University of Tokyo, underscored the importance of this breakthrough, stating, "Cas9 is at the very limit of this size restriction, so there has been a demand for a smaller Cas protein that can be efficiently packaged into AAV and serve as a genome-editing tool."
Cas9 is often not utilized alone, which is where the real issues begin. “(...) there have been issues with efficiency when [Cas9] is used for base editing and gene modulation where deaminase or transcription activation enzymes are fused to the Cas enzyme”, Satoshi Omura, the first author of the paper explains. “The compact AsCas12f variants we developed are less than one-third the size of Cas9. This means we can load the AAV with the AsCas12f along with deaminase or transcription activation enzymes with room to spare, potentially enabling efficient base editing and gene modulation."
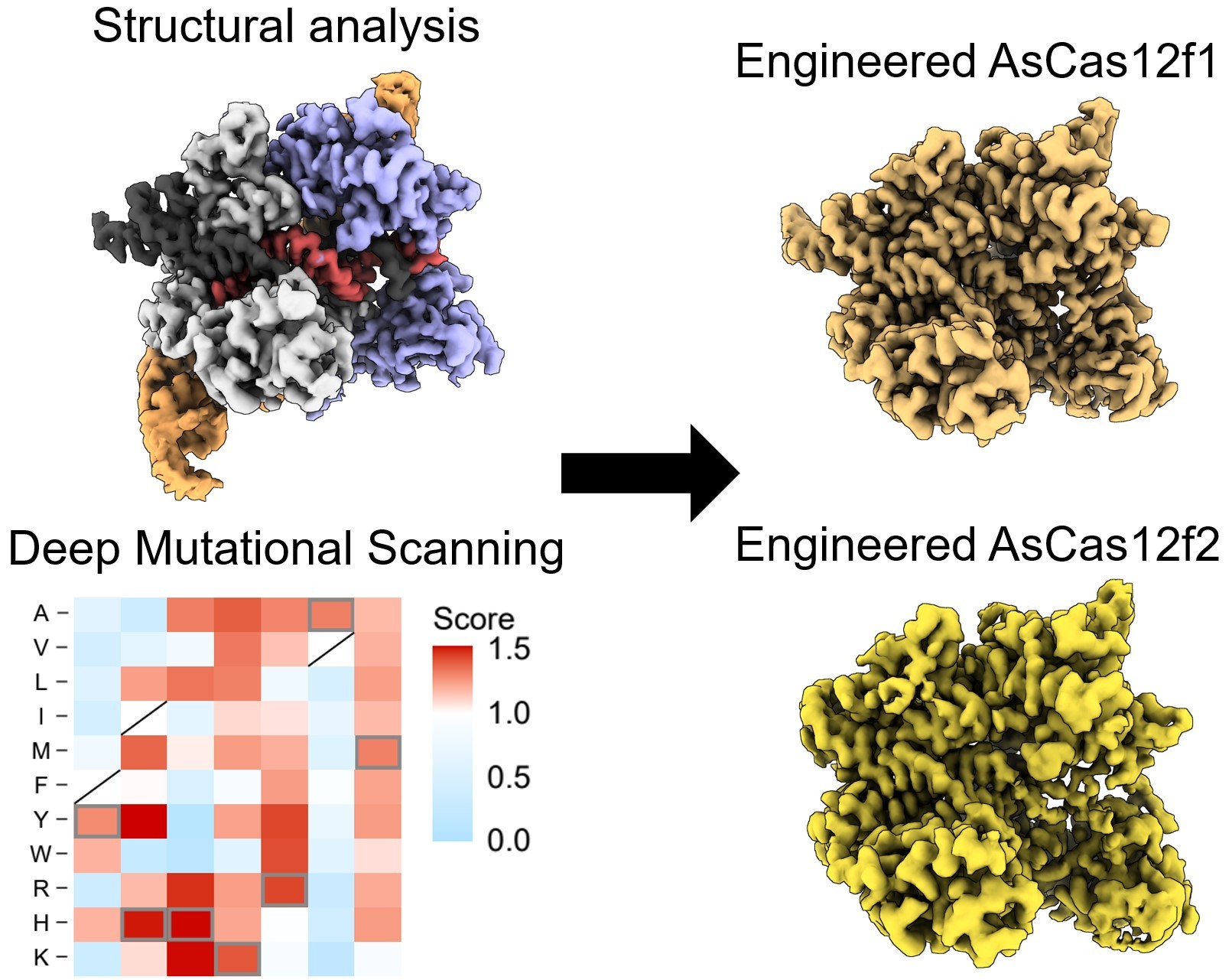
The journey towards the development of AsCas12f began with the identification of the enzyme in question, extracted from the bacteria Axidibacillus sulfuroxidans. However, initial tests revealed minimal genome-editing activity in human cells. Employing a technique called deep mutational scanning, the researchers embarked on a quest to optimize AsCas12f's performance.
Nureki elaborated on their approach, saying, "Using a screening method called deep mutational scanning, we assembled a library of potential new candidates by substituting each amino acid residue of AsCas12f with all 20 types of amino acids on which all life is based. From this, we identified over 200 mutations that enhanced genome-editing activity."
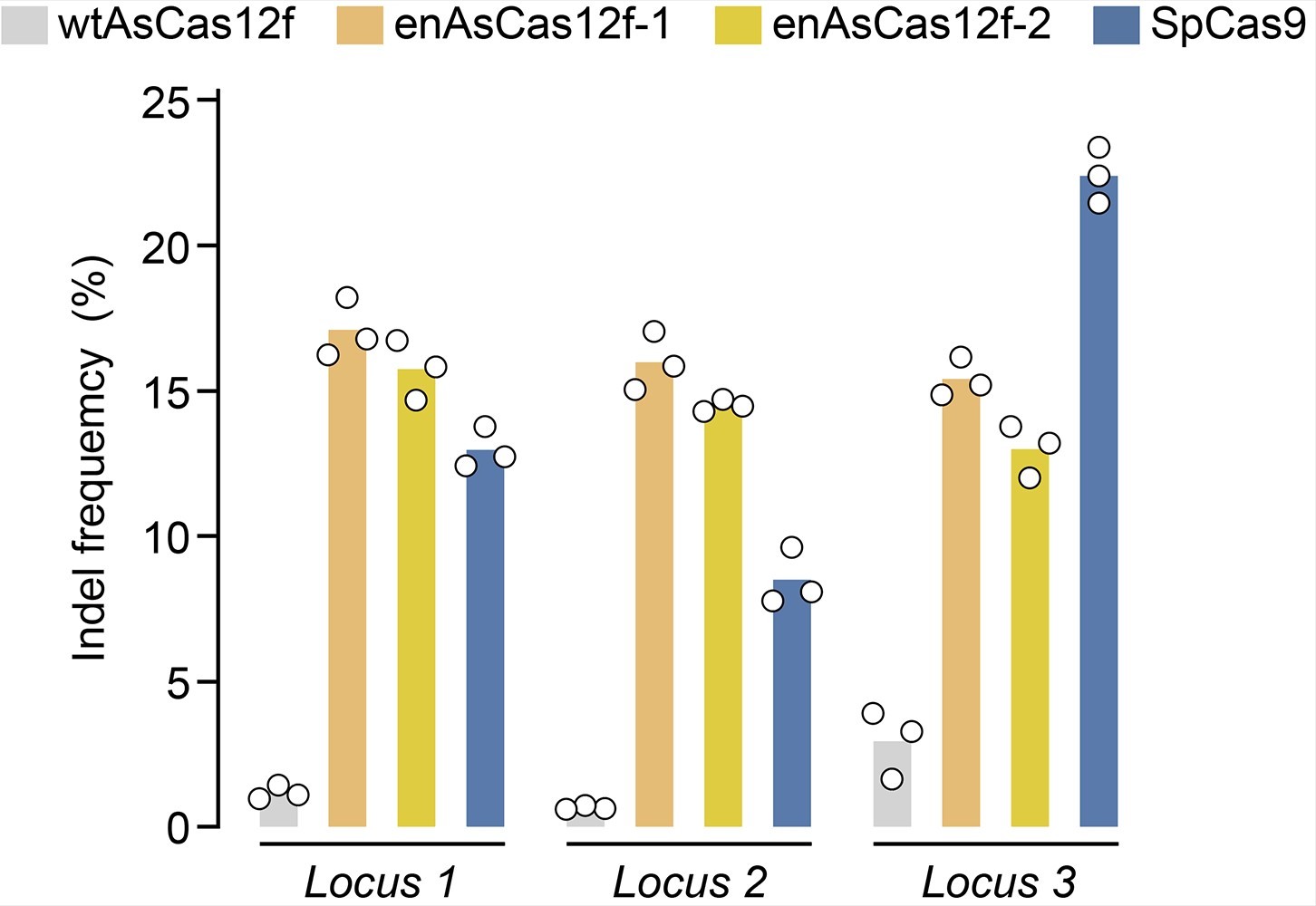
The true breakthrough came when the researchers combined these enhanced-activity amino acid mutations to create an engineered AsCas12f enzyme. This modified AsCas12f displayed a 10-fold increase in genome-editing activity compared to its unaltered counterpart, placing it on par with the widely-used Cas9, while retaining its compact size.
To demonstrate the practical applications of their innovation, the team conducted successful animal trials involving the engineered AsCas12f system. Live mice were administered with AsCas12f, alongside other genes, showcasing the potential for direct in-body treatments—a less time-consuming and costly alternative to traditional cell extraction, editing, and reinsertion methods.
The successful trials opened the door to the use of engineered AsCas12f in human gene therapies, with the potential to treat conditions like hemophilia, a genetic disorder characterized by abnormal blood clotting. However, with numerous possible combinations for improving the AsCas12f gene-editing system still on the table, researchers acknowledge that the selected mutations may not represent the optimal mix. “Based on our structural information and the mutational landscape from DMS, we hope to identify more optimal combinations of mutations through techniques like machine learning.” Omura stated.
As a forward-looking step, computational modeling and machine learning are being explored to sift through these combinations, potentially identifying even more potent improvements. "Elevating AsCas12f to exhibit genome-editing activity comparable to that of Cas9 is a significant achievement and serves as a substantial step in the development of new, more compact genome-editing tools," noted Nureki.
Emphasizing the ultimate goal of gene therapy, Nureki added, "For us, the crucial aspect of gene therapy is its potential to genuinely help patients. Using the engineered AsCas12f we developed, our next challenge is to actually administer gene therapy to aid people suffering from genetic disorders."
The unveiling of AsCas12f represents a watershed moment in genetic medicine, promising new avenues for more efficient and precise gene editing, offering hope to countless patients afflicted with genetic disorders. As the field continues to evolve, the potential for transformative breakthroughs in gene therapy looms large, furthering our quest to alleviate human suffering through cutting-edge scientific innovation.